-40%
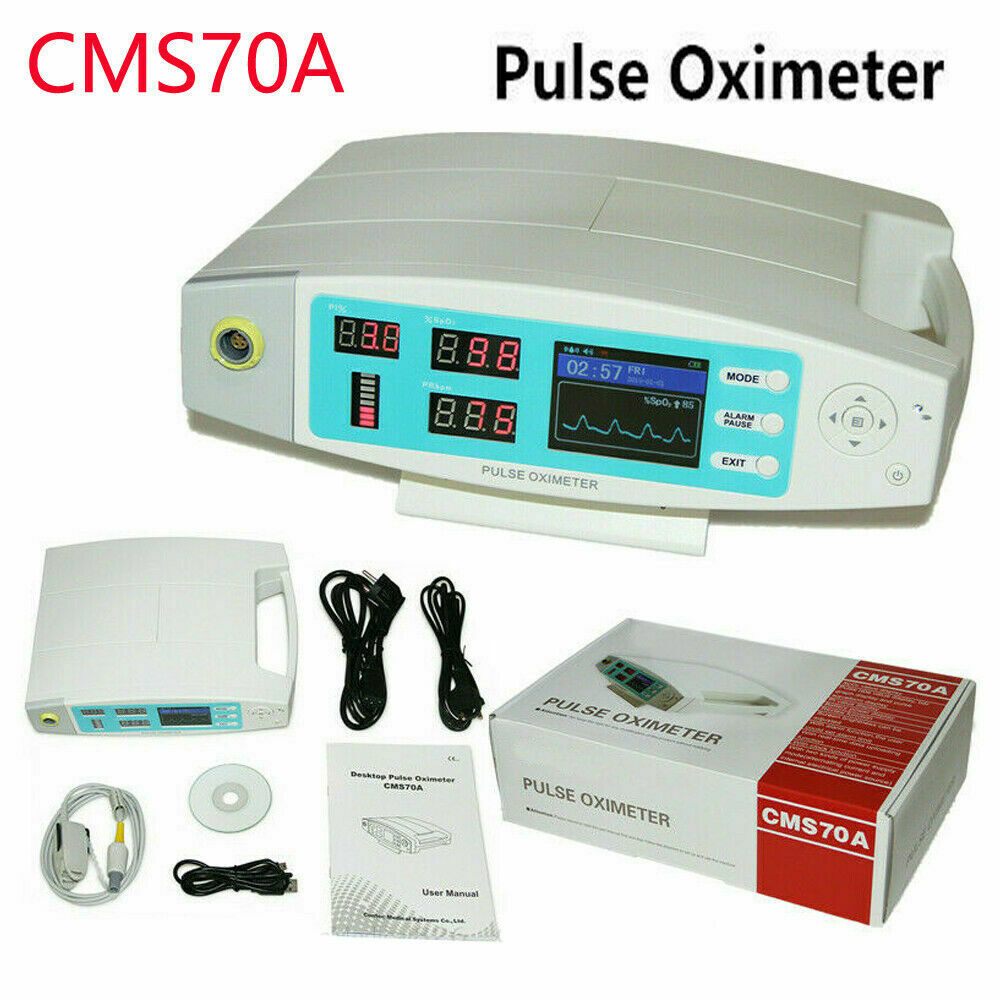
USA,CMS70A Desktop Pulse Oximeter Blood Oxygen HR PR SPO2 O2 Patient Monitor
$ 47.52
- Description
- Size Guide
Description
ambulatory blood pressure monitorContec CMS70A Pulse Oximeter
Description
Instructions
Principle of the CMS70A Pulse Oximeter is as follows: Photoelectric Oxyhemoglobin Inspection Technology is adopted in accordance with Capacity Pulse Scanning & Recording Technology,the Pulse Oximeter can be used in measuring the pulse oxygen saturation and pulse rate through finger.The product is suitable for being used in family, hospital, oxygen bar, community healthcare, physical care in sports (It can be used before or after doing sports, and it is not recommended to use the device during the process of having sport) and etc.
Major Features
Operation of the product is simple ,low power consumption
SpO2 value display
Pulse rate value display, bar graph display
PI display
Pulse waveform display
Menu operation
Screen brightness can be changed
The display mode can be changed
Pulse rate sound indication, it can be turned on or turned off
With measured data overruns limits and low-voltage alarm function,the upper/down alarm range can be adjustable
Battery capacity indication
Low-voltage indication: low-voltage indicator appears before working abnormally which is due to low-voltage, and with alarm function
Connected with an external oximeter probe
Data storage function,and the storage data can be uploaded to computer
Real-time data can be transmitted to computers
Main performance
Display Mode: TFT Screen and LED display
Screen Resolution: 320*240
SpO2 Measuring Range: 0%~100%(the resolution is 1%)
Accuracy: 70%~100%: ±2%, Below 70% unspecified.
PR Measuring Range: 30bpm~250bpm(the resolution is 1bpm)
Accuracy: ±2bpm or ±2% (select larger)
Measurement Performance in Weak Filling Condition:SpO2 and pulse rate can be shown correctly when pulse-filling ratio is 0.4%. SpO2 error is ±4%, pulse rate error is ±2 bpm or ±2% (select larger).
Resistance to surrounding light: The deviation between the value measured in the condition of man-made light or indoor natural light and that of darkroom is less than ±1%.
Power Consumption: About 350mA
Voltage:AC 100~240V, 50/60HZ
Power Supply: Built-in recharge Li-Polymer
Battery working hour: after the device built-in battry finished the charge,it's continuous working time is not less than 1 hour
Safety Type:II device, BF Type
Accessories
Sell in standard
A user manual
A power wire
A data line
An oximeter probe
A disk (PC software)
Sell in addition
Other oximeter probe(Refer to the product configuration BOM for details)
Physical Identity
Dimension:269(L)×222(W)×79(H)mm
Weight: About 1Kg
Payment
1.We only accept payment to us through Paypal .Paypal is the safest and quickest way to pay online and offers seller and buyer protection.
2.Please pay within 2 days so as to avoid unnecessary delays and to ensure you get your item ASAP.
3.Buyers will receive the transaction notification from eBay within 24 hours. We reserve the rights to cancel the order if the buyer does not pay us or contact us for payment within 7 business days after auction end.
4.Payment must be received unless buyer has contacted us with any specific reasons. An unpaid strike will be sent if the item has not been paid after 14 days.
Shipping
1.Our shipping rates include the cost of shipping, handling, packaging,custom fees are not included,buyers pay the taxes.
2.We usually shipped out the items the next business day after payment has been verified (Sat. & Sun. excluded.)
3.We ship items to USA/AU/CA/IT/FR/MX/SG/NL/PO/IE/PT by E-Package, other country by China Air mail and Singapore Airmail. It Usually take about 5-25 business days.
Terms of Sale
Buy safe Products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies.If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item. If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE,TUV of Europe.The Fingertip Pulse Oximeter that is FDA 510K Approved.
About Us
Contec Medical Systems focusing on research, manufacture and distribution of medical instruments,was founded in 1992 as a high-tech company.At present there are more than 1200 employees in our company.Our product line covers a wide range of 13 categories.Most of the domestic hospitals are our customers.Contec hopes to cooperate with international companies to supply more innovative design and advanced technology products We sincerely welcome you to become one of our global partners.We are looking forward to establishing a successful business relationship with you.
Contact Us
Please contact us first if you are not satisfied with the item when you receive it or haven't received item after 30 days, We will try our best to solve any problem. Open case and negative feedback is not conducive to solve the problem for both sides. hope you can understand!
All emails will be answered within 24 hours on weekdays(but within 48hours on weekends).Please don't hesitate to contact us with any queries or comments about our items or your problem.
If you do not receive our reply, please kindly re-sent your email and we will reply to you as soon as possible.
Only English user guide, if you need any other language, please contact us!